
Sodium Cyanide (NaCN) is a compound renowned for its high toxicity and is frequently utilized in industries such as mining, electroplating, and chemical synthesis. Given its hazardous nature, understanding its behavior, especially the potential for direct volatilization into the air, is of utmost importance for safety and environmental protection.
Chemical Properties of Sodium Cyanide
Sodium cyanide is a white, crystalline solid with a high melting point of 563.7 °C and a boiling point of 1496 °C. Under normal ambient conditions, typically characterized by temperatures ranging from 20 - 25 °C and standard atmospheric pressure, it exists in a solid state. From a basic thermodynamic perspective, substances generally require energy input to transition from a solid or liquid state to a gaseous state (volatilization). Since the ambient temperature is far below the boiling point of sodium cyanide, it does not readily convert into a gas spontaneously under normal circumstances.
Factors Affecting Volatilization
However, the stability of Sodium Cyanide can be affected by various factors. One significant factor is its reaction with water and acids. When Sodium cyanide comes into contact with water, it hydrolyzes to form hydrogen cyanide (HCN), a highly volatile and extremely toxic gas. The chemical reaction can be represented as follows:
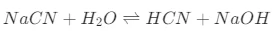
The equilibrium of this reaction is influenced by the pH of the solution. In acidic environments, the reaction is more likely to proceed in the forward direction, promoting the formation of HCN. For example, if sodium cyanide is exposed to acidic rain or acidic industrial wastewater, a rapid generation of HCN gas may occur, which then volatilizes into the air.
Another factor is temperature elevation. Although ambient temperatures are insufficient to cause direct volatilization of sodium cyanide itself, in industrial accidents or improper storage situations where local temperatures may rise significantly, the kinetic energy of sodium cyanide molecules increases. This could potentially lead to an increased rate of hydrolysis reactions and, consequently, a higher likelihood of HCN formation and volatilization.
Safety and Environmental Implications
The potential for the formation and volatilization of HCN from sodium cyanide has significant safety and environmental implications. In the workplace, if sodium cyanide is not properly stored or handled, accidental exposure to moisture or acids could result in the release of HCN gas, posing a severe threat to the health of workers. Symptoms of HCN exposure can range from mild respiratory distress and dizziness to life - threatening situations such as cardiac arrest and coma.
In the environment, the release of HCN from sodium cyanide can contaminate air, soil, and water bodies. It can harm various organisms, disrupt ecosystems, and accumulate in the food chain, further exacerbating the environmental damage.
Prevention and Mitigation
To prevent the volatilization - related risks of sodium cyanide, strict safety protocols must be adhered to. Sodium cyanide should be stored in a dry, cool, and well - ventilated area, away from sources of moisture and acids. In industrial applications, proper handling procedures, including the use of appropriate personal protective equipment (PPE) and the installation of gas detection systems, are essential. In case of a spill or accidental release, immediate containment measures should be taken, and neutralization with appropriate alkaline solutions may be necessary to prevent the formation of HCN.
In conclusion, while sodium cyanide does not directly volatilize into the air under normal ambient conditions, it can indirectly lead to the release of volatile and toxic HCN gas through hydrolysis reactions under specific circumstances such as contact with water or acids, or elevated temperatures. Understanding these mechanisms is crucial for ensuring the safe handling, storage, and use of sodium cyanide, as well as for protecting human health and the environment.
- Random Content
- Hot content
- Hot review content
- Toxicity Assessment of Sodium Cyanide and Relevant Hazard Prevention Measures
- Industrial Grade Sodium Metabisulfite 96.5%
- Powdery emulsion explosive
- Colloidal emulsion explosive
- Ammonium Chloride 99.5% Mining Collector
- Ammonium Nitrate Porous Prills
- Potassium Permanganate – Industrial Grade
- 1Discounted Sodium Cyanide (CAS: 143-33-9) for Mining - High Quality & Competitive Pricing
- 2Sodium Cyanide 98% CAS 143-33-9 gold dressing agent Essential for Mining and Chemical Industries
- 3Sodium Cyanide 98%+ CAS 143-33-9
- 4Anhydrous Oxalic acid 99.6% Industrial Grade
- 5Soda Ash Dense / Light 99.2% Sodium Carbonate Washing Soda
- 6Oxalic acid for mining 99.6%
- 7Calcium hydroxide Industrial Grade 90%
- 1Sodium Cyanide 98% CAS 143-33-9 gold dressing agent Essential for Mining and Chemical Industries
- 2High Quality 99% Purity of Cyanuric chloride ISO 9001:2005 REACH Verified Producer
- 3 High-Quality Sodium Cyanide for Leaching
- 4Powdery emulsion explosive
- 5Industry Grade Electron grade 98% Sulfuric Acid H2SO4 Sulphuric Acid Battery Acid Industrial Sulfuric Acid
- 6Colloidal emulsion explosive
- 7sodium hydrosulfide 70% flakes used Mining Industry











Online message consultation
Add comment: